
About us

One team - one mission
With years of experience at leading companies and research institutions, we are committed to developing innovative medical devices that improve patient care.
Our Medical Board provides expert guidance and support for our engineering projects and clinical studies.
Shaping the future of brain monitoring
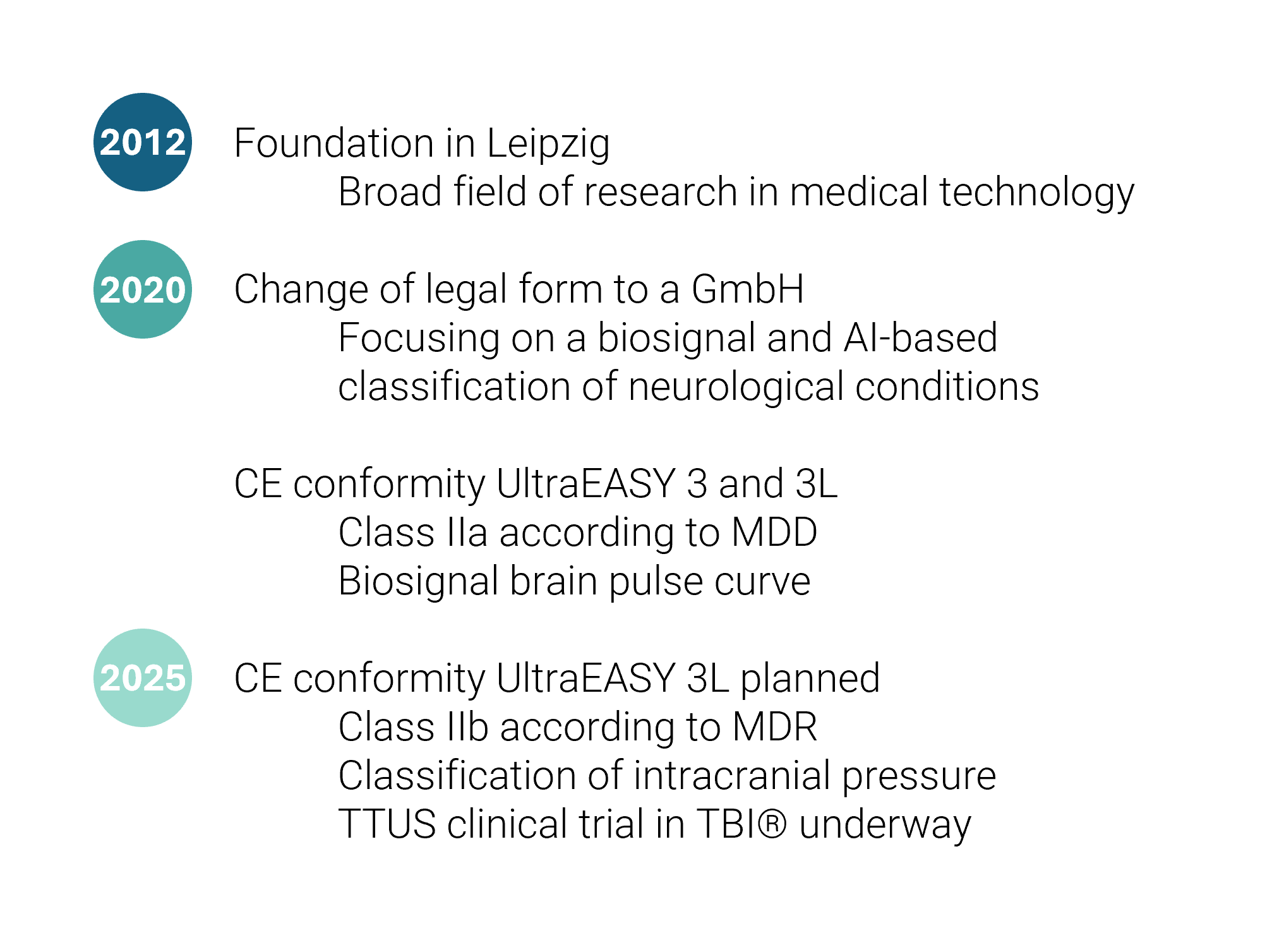
The successor to the UltraEASY 3L, TTUS in TBI®, is currently undergoing clinical trials. This is the first medical device to non-invasively classify intracranial pressure, with a planned release in 2025.
*Transcranial transmission ultrasound, TTUS®

Partnering for innovation in patient care
This includes start-up and MedTech platforms that promote the exchange of expertise and experience. Our suppliers provide high-quality, state-of-the-art hardware components for our medical products, while we develop application-oriented solutions through research collaborations with Fraunhofer Institutes and technical universities.
Certificates
We are an ISO 13485 certified medical technology manufacturer, ensuring the highest quality and safety standards for our devices. All our medical devices bear the CE mark, demonstrating compliance with European Union regulations.
Download our certificates here.