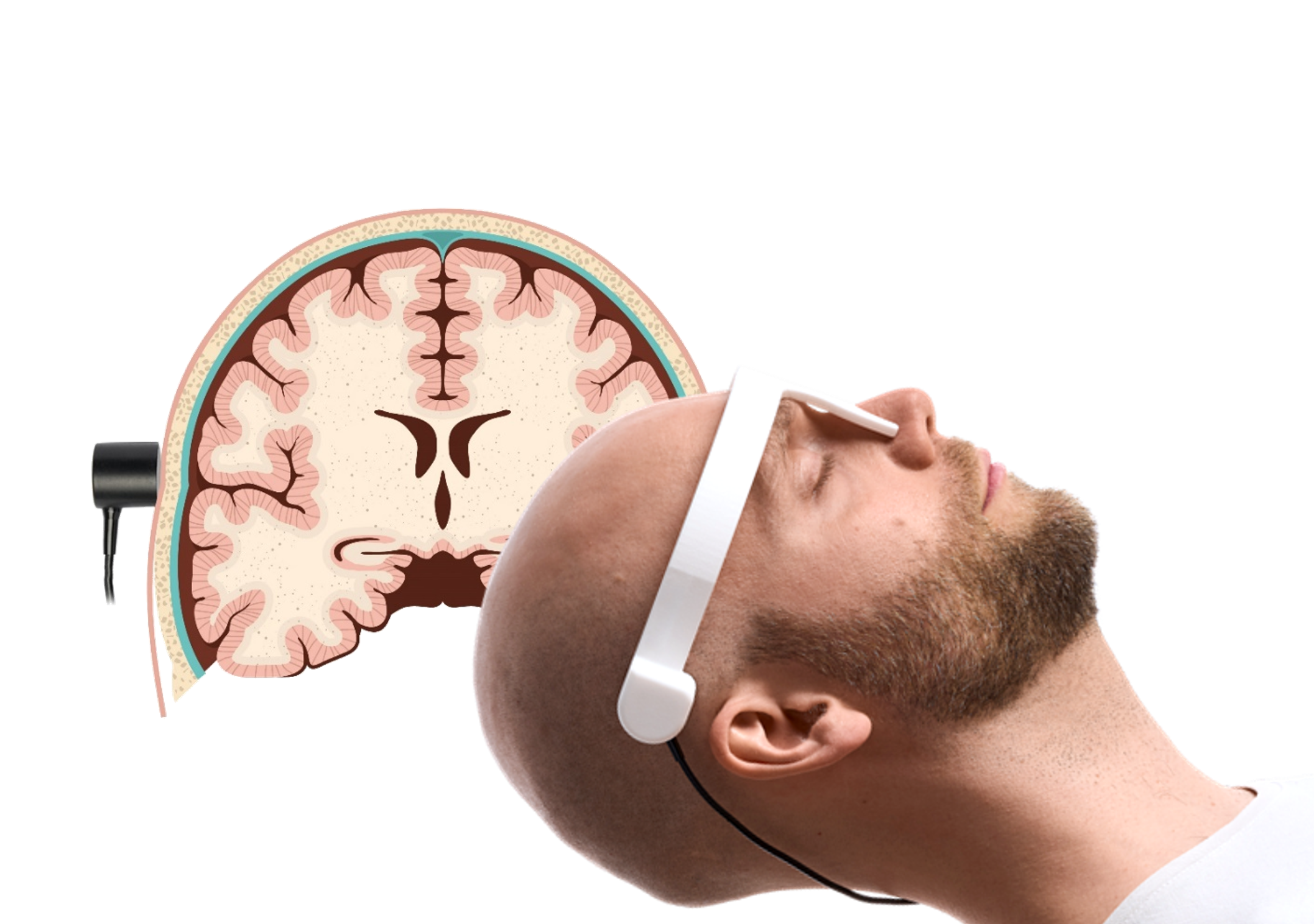
Transcranial Transmission Ultrasound, TTUS®
UltraEASY 3L
Non-invasive Assessment of Intracranial Compliance
Time is Brain
Each day, 650 people in Germany suffer a traumatic brain injury. More than 2,700 patients die each year from its consequences. Every second counts when it comes to recognizing and treating the damage caused by an intracranial injury at an early stage. Direct measurement of intracranial pressure, however, requires an invasive procedure with a significant complication rate of 20%.*
*Source: TraumaRegister DGU®
Measurement
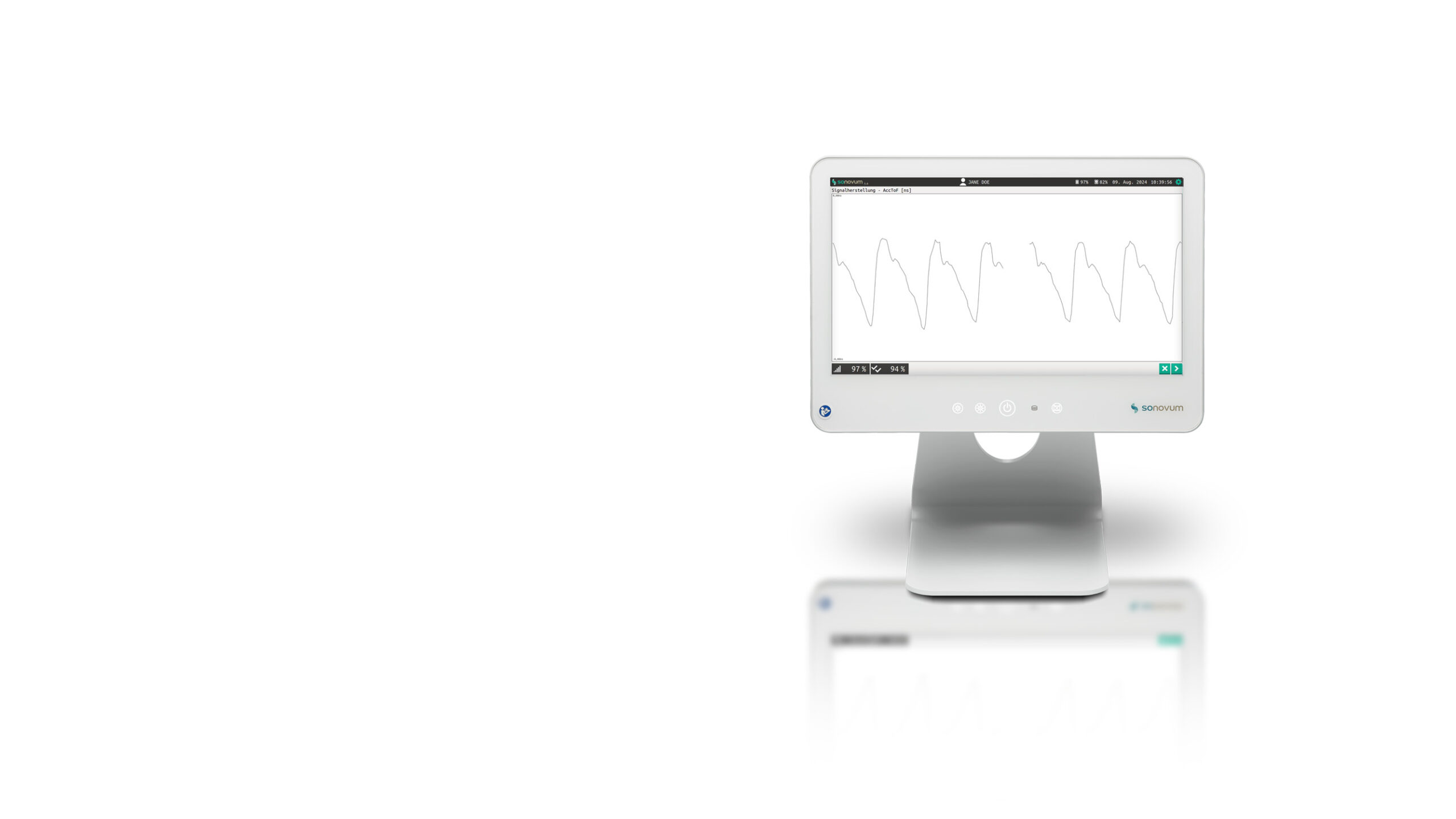
The UltraEASY 3L medical device allows for non-invasive assessment of the Brain Pulse Curve biosignal in just a few minutes.
Brain Pulse Curve
UltraEASY 3L utilizes transcranial ultrasound (TTUS) to non-invasively assess intracranial compliance. The device emits an ultrasound signal that passes through the patient's head, capturing a representative sample volume of blood vessels, brain tissue, and cerebrospinal fluid. This data is processed and displayed as a Brain Pulse Curve, a 100 Hz ultrasound time-of-flight waveform.
Intracranial Compliance
Analysis of the Brain Pulse Curve allows for differentiation between normal and reduced intracranial compliance. Clinical evaluation is underway to establish the Brain Pulse Curve as a reliable indicator of ICP. This non-invasive approach has the potential to identify patients with elevated ICP, allowing for early intervention and potentially reducing the need for neurosurgical procedures.
Clinical Studies and Validation
Since receiving CE certification according to MDD IIa in 2020, the UltraEASY 3L medical device has been a reliable data platform for clinical studies, with comprehensive technical evaluations confirming its sensitivity, time resolution, sound field, and safety.
- Observational studies at university hospitals
- IIT study at the University Hospital Munich, TUM Rechts der Isar 2022/2023
- Clinical trial for non-invasive ICP classification, CE MDR IIb, ongoing
Use in Clinical Practice
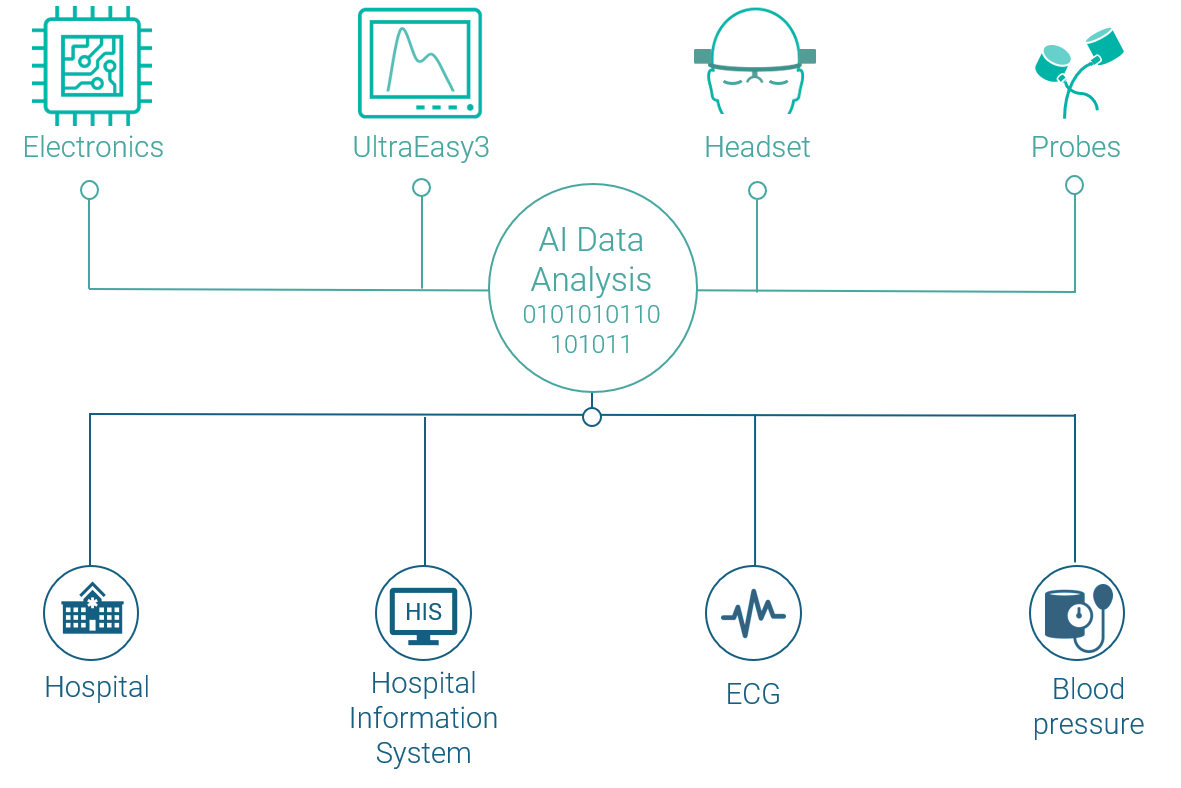
UltraEASY 3L is compatible with various clinical monitoring and analysis systems, making it easy to integrate the medical device into your daily work. With UltraEASY 3L, you get an interoperable solution that allows you to examine clinical biosignals and parameters such as blood pressure, ECG or ventilation modes in different multimodal setups in a time-synchronized manner.
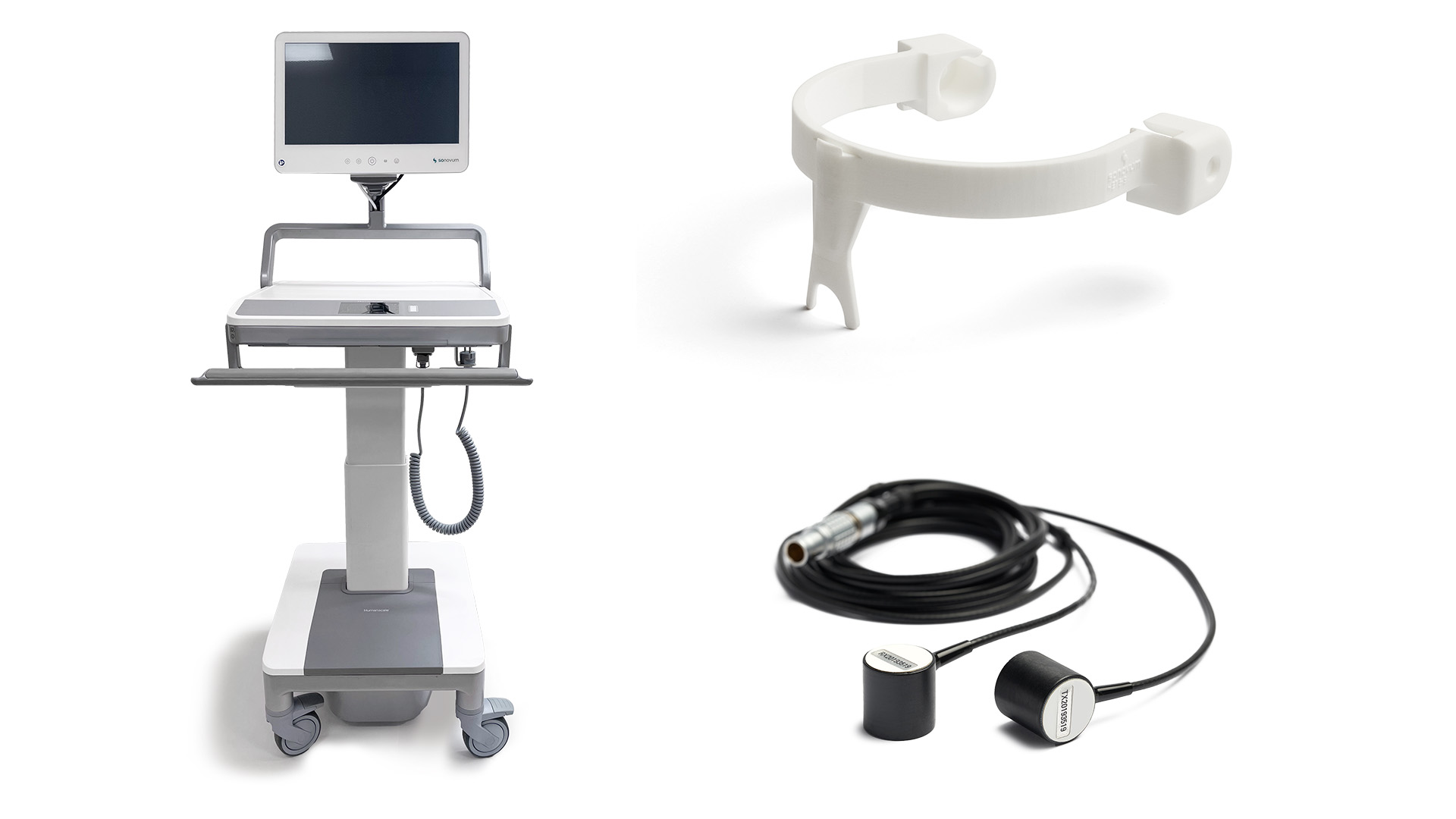
Basic Equipment
The UltraEASY 3L offers flexible setup options with both mobile (trolley) and tabletop (stand) versions available. It can be fixed to an individual location using the integrated VESA mount. The device includes ultrasound probes and multiple sizes of headsets, enabling quick transducer changes. UltraEASY 3L can be integrated with your existing patient monitoring systems through supported communication interfaces.
Our team will be happy to advise you on individual configuration options for research and clinical practice.