
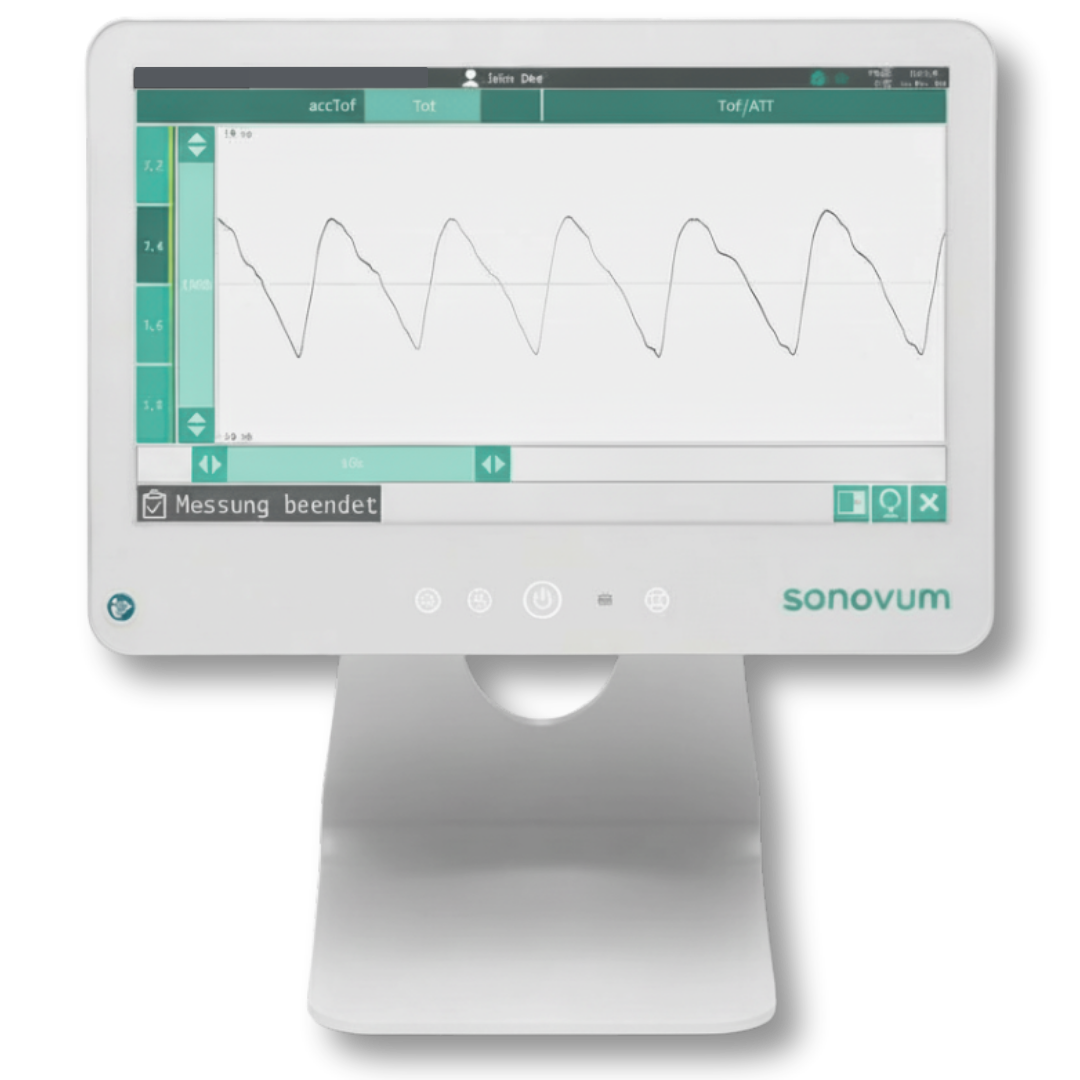

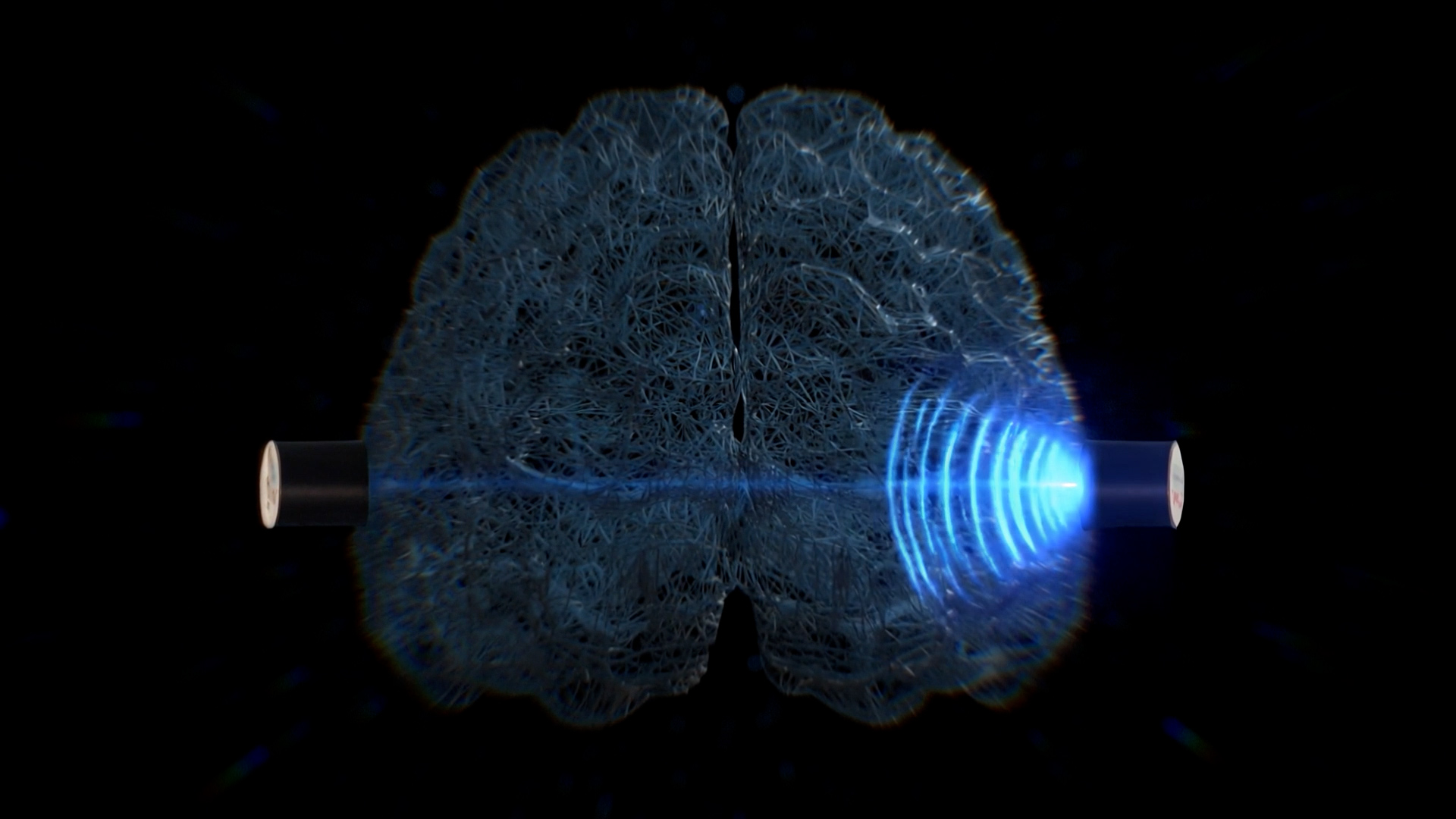
The device is in advanced clinical validation and progressing through the evidence generation required for CE marking under MDR. Completion of the pivotal study is expected in February 2026, in alignment with European and internationally recognized regulatory standards.
We start with one clearly defined first use case, but the potential of non-invasive intracranial pressure assessment goes far beyond. Our goal is to build a new category in brain diagnostics and to expand step by step into additional neurological and emergency-care applications, where fast, safe brain assessment can change clinical decisions and outcomes.